part of the material in the opposite direction. The
TORSIONAL STRESS
action of a pair of scissors is an example of shear
forces and shear stresses. The scissors apply shear
Torsional stresses develop in a material when
forces, and the material being cut resists the shear
external forces are applied in such a way that they tend
forces by its internal shear stresses. Forces tending to
to produce rotation. A ship's shaft, for example,
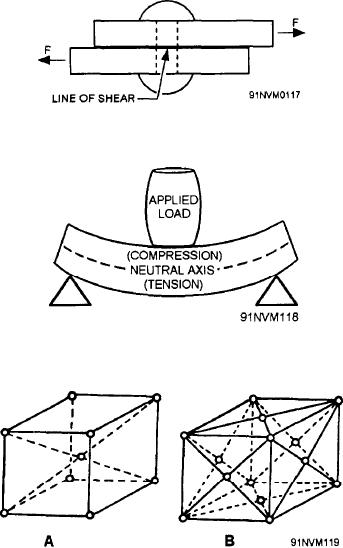
produce shear in a rivet are illustrated in figure 6-3.
rotates when the external applied forces are greater
Shear stresses are not shown, since they are
than the internal torsional stresses developed in the
considerably more complex than tension stresses and
shaft. Torsional stress is primarily a special form of
compression stresses.
shear stress, although it may also involve some
compression stress and some tension stress.
BENDING STRESS
INTERNAL STRUCTURE OF METALS
Bending stresses develop when a material is
subjected to external forces that tend to bend it. When
The atoms in all solid metals are arranged in some
a load is applied to a beam, for example, as shown in
definite geometric (or crystallographic) pattern. The
figure 6-4, the upper surface is in compression and the
smallest grouping of atoms that has the complete
lower surface is in tension. The NEUTRAL AXIS,
symmetrical arrangement of the crystal is called a unit
indicated by the broken line in figure 6-4, is neither in
cell. The regular arrangement of these atoms is called
compression nor in tension.
a space lattice. A unit cell is much too small to be seen.
When a great many unit cells are combined, however,
they form a visible crystal that has the same geometric
structure as the unit cell.
A number of different geometrical arrangements
of atoms are possible, but most metals have space
lattices that are basically shaped like cubes, tetragons,
or hexagons. Figure 6-5 shows the body-centered
Figure 6-3.--Shearing forces applied to a rivet.
cubic and face-centered cubic space lattices.
How do crystals form? When the metal is in the
liquid state, the atoms move freely and are not
arranged in any orderly fashion. When the metal
begins to cool, however, the atoms move more and
more slowly. When the freezing (solidifying)
temperature of the metal is reached, the atoms begin
to form unit cells of the type characteristic of the
particular metal. In this crystallization process, the
atoms give up energy in the form of heat. As this
energy flows from the metal, other atoms form around
Figure 6-4.--Load applied to a beam.
each of the original unit cells in a definite pattern. This
definite and repeating pattern upon solidification is
called a space lattice. Eventually all of the metal is
changed from the liquid state, in which the atoms are
moving freely, to the solid state, in which the atoms
are arranged in a definite, orderly pattern. At this
point, we say that the metal has completely solidified
or frozen.
If crystallization could proceed without any
the external form of the internal space lattice. As a
rule, however, the space lattices do not all line up
Figure 6-5.--Crystal structure of iron. A. Body-centered cubic,
perfectly with each other; this means that the growth
9-atom space lattice. B. Face-centered cubic, 14-atom space
of some crystals interferes with the growth of others.
lattice.
6-4