have the typical shape of the space lattice, it is custom-
In other words, space lattices that are not oriented in
ary to call each visible unit a grain rather than a crystal.
approximately the same way cannot join each other.
The areas between adjacent grains are shown as grain
As a result, the crystallization process usually results
boundaries. The grain boundary material has somewhat
in the growth of many small crystals rather than one
different properties than the actual grains or crystals;
large one. In any given piece of solid metal, the size
this is partly because the space lattices are distorted at
of the crystals will vary. The larger ones are the result
the grain boundaries and partly because the process of
of the combination of a great many space lattices that
crystallization tends to push impurities out of the crys-
happened to line up in such a way that they could join
tals and into the grain boundaries.
each other.
The term grain structure refers to the crystalline
Because the crystals interfere with each other as
structure of the metal, often with particular reference to
they grow, a piece of metal in cross section may show
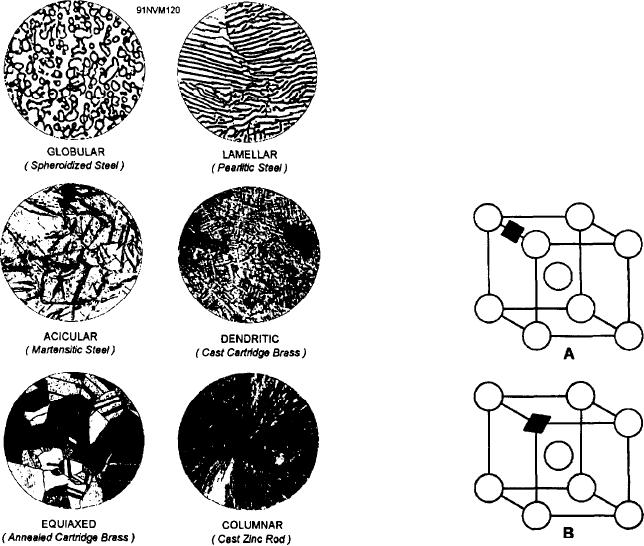
the shape and size of the grains. Figure 6-6 illustrates
very few characteristic crystal shapes. Note, however,
several types of gram structure, as seen under a micro-
that the metal is still considered crystalline even if the
scope. The size of the grains depends upon a number of
crystalline forms are distorted. The crystals are there,
factors, including the nature of the metal, the tempera-
but they are not usually perfect in shape.
ture to which it is heated, the length of time it is held at
When a metal crystallizes in such a way that the
a specified temperature, and the rate at which it is cooled
crystals are not perfectly formed and therefore do not
from a liquid to the solid state. In general, the quicker a
metal solidifies, the smaller the grain will be. The size
of grain structure desired for any particular application
depends upon the purpose for which the metal is to be
used.
In alloys, the internal structure may be in the form
of crystals of pure metals, solid solutions, intermetallic
compounds, mechanical mixtures, or some combina-
tion of these structures.
In a solid solution, the elements are completely
dissolved in each other, with the atoms of each
element fitting into and forming part of the space
lattice of the other element. Figure 6-7 illustrates two
Figure 6-7.--Space lattices of two forms of solid solution. A.
Atoms of one element fit between atoms of another element.
B. Atoms of one element replace atoms of another element.
Figure 6-6.--Grain structure in metals.
6-5