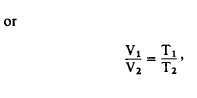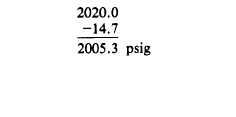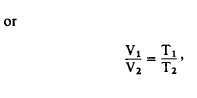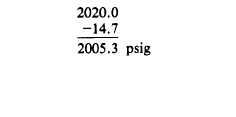Example of Boyle’s law: 4 cubic feet of
nitrogen are under a pressure of 100 psi (gauge).
The nitrogen is allowed to expand to a volume
of 6 cubic feet. What is the new gauge pressure?
Remember to convert gauge pressure to absolute
pressure by adding 14.7.
Using equation 11-6, V1P1 = V2P2, where V1 is
4 ft3, V2 is 6 ft, and P1 is 100 psig:
CHARLES’S LAW
Boyle’s law assumes conditions of constant
temperature. In actual situations this is rarely the
case. Temperature changes continually and affects
the volume of a given mass of gas.
Jacques Charles, a French physicist, provided
much of the foundation for the modern kinetic
theory of gases. Through experiments, he found
that all gases expand and contract proportionally
to the change in the absolute temperature,
providing the pressure remains constant. The
relationship between volume and temperature is
known as Charles’s law. It states: The volume of
a gas is proportional to its absolute temperature,
if constant pressure is maintained. In equation
form, this relationship may be expressed as
Equation 11-7
where V1 and V2 are the original and final
volumes, and T1 and T2 are the original and final
absolute temperatures.
Since an increase in the temperature of a gas
causes it to expand if the pressure is kept constant,
it is reasonable to expect that if a given sample
is heated within a closed container and its volume
remains constant, the pressure of the gas will
increase. Experiments have proven this to be true.
In equation form, this becomes
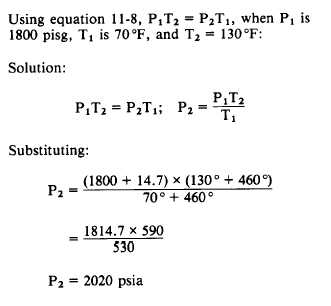
P1T2 = P2T1
Equation 11-8
or
This equation states that for a constant volume,
the absolute pressure of a gas varies directly with
the absolute temperature.
Example: A cylinder of gas under a pressure
of 1800 psig at 70°F is left out in the sun in the
tropics and heats up to a temperature of 130°F.
What is the new pressure within the cylinder?
(Remember that both pressure and temperature
must be converted to absolute pressure and
absolute temperature.)
Converting absolute pressure to gauge pressure:
11-5