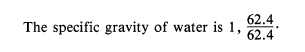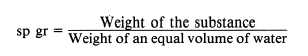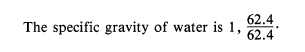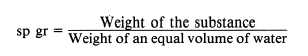a circular cross section when it is filled with water
under pressure. The outward push of the water
is equal in every direction.
So far we have explained the effects of
atmospheric pressure on liquids and how external
forces are distributed through liquids. Let us now
focus our attention on forces generated by the
weight of liquids themselves. To do this, we must
first discuss density, specific gravity, and Pascal’s
law.
Density and Specific Gravity
The density of a substance is its weight per unit
volume. The unit volume in the English system
of measurement is 1 cubic foot. In the metric
system it is the cubic centimeter; therefore, density
is expressed in pounds per cubic foot or in grams
per cubic centimeter.
To find the density of a substance, you must
know its weight and volume. You then divide its
weight by its volume to find the weight per unit
volume. In equation form, this is written as
Equation 2-4.
EXAMPLE: The liquid that fills a certain
container weighs 1,497.6 pounds. The
container is 4 feet long, 3 feet wide, and
2 feet deep. Its volume is 24 cubic feet
(4 ft x 3 ft x 2 ft). If 24 cubic feet of this
liquid weighs 1,497.6 pounds, then 1 cubic
foot weighs
or 62.4 pounds. Therefore, the density of
the liquid is 62.4 pounds per cubic foot.
This is the density of water at 4°C and is
usually used as the standard for comparing
densities of other substances. The temperature of
4°C was selected because water has its maximum
density at this temperature. In the metric system,
the density of water is 1 gram per cubic
centimeter. The standard temperature of 4°C is
used whenever the density of liquids and solids
is measured. Changes in temperature will not
change the weight of a substance but will change
the volume of the substance by expansion or
contraction, thus changing the weight per unit
volume.
In physics, the word specific implies a ratio.
Weight is the measure of the earth’s attraction for
a body. The earth’s attraction for a body is called
gravity. Thus, the ratio of the weight of a unit
volume of some substance to the weight of an
equal volume of a standard substance, measured
under standard pressure and temperature con-
ditions, is called specific gravity. The terms
specific weight and specific density are sometimes
used to express this ratio.
The following formulas are used to find the
specific gravity (sp gr) of solids and liquids, with
water used as the standard substance.
or,
The same formulas are used to find the specific
gravity of gases by substituting air, oxygen, or
hydrogen for water.
If a cubic foot of a certain liquid weighs 68.64
pounds, then its specific gravity is 1.1,
Thus, the specific gravity of the liquid is the
ratio of its density to the density of water. If the
specific gravity of a liquid or solid is known, the
density of the liquid or solid maybe obtained by
multiplying its specific gravity by the density of
water. For example, if a certain hydraulic liquid
has a specific gravity of 0.8, 1 cubic foot of the
liquid weighs 0.8 times as much as a cubic foot
of water—0.8 times 62.4, or 49.92 pounds. In the
metric system, 1 cubic centimeter of a substance
with a specific gravity of 0.8 weighs 1 times 0.8,
or 0.8 grams. (Note that in the metric system the
specific gravity of a liquid or solid has the same
numerical value as its density, because water
weighs 1 gram per cubic centimeter.)
Specific gravity and density are independent
of the size of the sample under consideration and
depend only on the substance of which it is made.
A device called a hydrometer is used for
measuring the specific gravity of liquids.
2-4