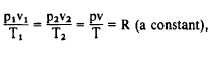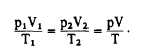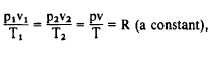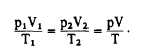CHAPTER 11
PNEUMATICS
The word pneumatics is a derivative of the
Greek word pneuma, which means air, wind, or
breath. It can be defined as that branch of
engineering science that pertains to gaseous
pressure and flow. As used in this manual,
pneumatics is the portion of fluid power in which
compressed air, or other gas, is used to transmit
and control power to actuating mechanisms.
This chapter discusses the origin of pneu-
matics. It discusses the characteristics of gases and
compares them with those of liquids. It also
explains factors which affect the properties of
gases, identifies and explains the gas laws, and
identifies gases commonly used in pneumatics and
their pressure ranges. It also discusses hazards of
pneumatic gases, methods of controlling contami-
nation, and safety precautions associated with
compressed gases.
DEVELOPMENT OF PNEUMATICS
There is no record of man’s first uses of air
to do work. Probably the earliest uses were to
separate chaff from grain and to move ships. One
of the first pneumatic devices was the blow gun
used by primitive man. In the latter part of the
eighteenth century, heated air was used to carry
the first balloon aloft. The heated air, being
lighter than the surrounding air, caused the
balloon to rise.
Every age of man has witnessed the develop-
ment of devices which used air to do work.
However, man used air to do work long before
he understood it.
Many of the principles of hydraulics apply to
pneumatics. For example, Pascal’s law applies to
gases as well as liquids. Also, like hydraulics, the
development of pneumatics depended on closely
fitted parts and the development of gaskets and
packings. Since the invention of the air com-
pressor, pneumatics has become a very reliable
way to transmit power.
Probably one of the most common uses of
pneumatic power is in the operation of pneumatic
tools. However, you should understand that
pneumatics is also of great importance in large
and complex systems such as the controls of vital
propulsion and weapon systems.
CHARACTERISTICS OF GASES
Recall from chapter 1 that gas is one of the
three states of matter. It has characteristics similar
to those of liquids in that it has no definite shape
but conforms to the shape of its container and
readily transmits pressure.
Gases differ from liquids in that they have no
definite volume. That is, regardless of the size or
shape of the containing vessel, a gas will
completely fill it. Gases are highly compressible,
while liquids are only slightly so. Also, gases are
lighter than equal volumes of liquids, making
gases less dense than liquids.
DENSITY
Early experiments were conducted concerning
the behavior of air and similar gases. These
experiments were conducted by scientists such as
Boyle and Charles (discussed later in this chapter).
The results of their experiments indicated that the
gases’ behavior follows the law known as the
ideal-gas law. It states as follows: For a given
weight of any gas, the product of the absolute
pressure and the volume occupied, divided by the
absolute temperature, is constant. In equation
form, it is expressed as follows:
Equation 11-1
For 1 pound of gas,
Equation 11-2
11-1