transformations to be completed in the normal manner,
resulting in quite different structures than those
obtained by very slow cooling of the same material.
Remember this fact about cooling-if you do not cool
a metal properly, you will not get the desired results, no
matter how carefully you heat and soak the metal. In
addition, very rapid cooling can cause internal stresses
in your material, which may lead to cracking.
In plain-carbon steel, the properties of the material
are largely determined by the form and distribution of
the ferrite and the cementite. Most heat treatment of
plain-carbon steels consists of heating the material
slightly above its transformation temperature, holding
it at this temperature until it is completely austenitic,
and then cooling it at the rate required to produce a
particular kind of structure. Thus austenite, a solid
solution of carbon and gamma iron, might be
considered the basis from which all plain-carbon steel
structures are derived.
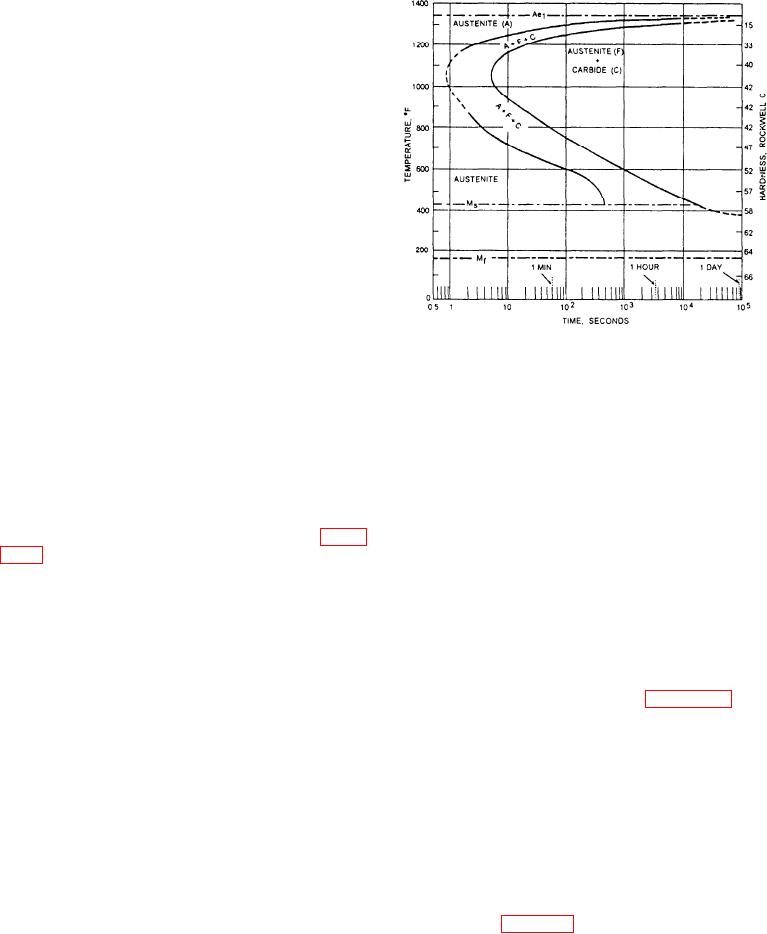
Figure 15-15.--Isothermal transformation diagram (S-curve)
As noted before, the very slow cooling of austenite
for eutectoid carbon steel.
to room temperature produces structures that are
salts. Occasionally, solid materials are used as
combination depends upon the percentage of carbon in
quenching mediums. In each instance, the quenching
the ahoy. With less than about 0.83 percent carbon, the
medium and the quenching procedure must be selected
structure is a combination of ferrite and pearlite. With
on the basis of the nature of the material being treated,
just about 0.83 percent carbon, the structure is entirely
the size and design of the piece, and the properties that
pearlitic. With more than 0.83 percent carbon, the
are required in the final product.
structure is a combination of pearlite surrounded by
Isothermal Transformation
cementite at the grain boundaries as shown in figure
The course of transformation of austenite when the
If the steel is cooled rapidly, a different structure
steel is quenched to and held at various constant
will result. The austenite will be retained until the
elevated temperature levels (isothermal transformation)
material reaches a temperature of about 430F. At this
is shown by a diagram known as the isothermal
point the transformation from austenite to a structure
transformation diagram (I-T diagram). This diagram is
called MARTENSITE begins. Martensite is a very hard
also called the Bain S-curve or the TTT diagram, for
and highly stressed structure. It is formed at the moment
time, temperature, and transformation. Such a diagram
gamma iron changes to alpha iron. Since gamma iron
can hold a great deal more carbon in solid solution than
I-T diagram of a steel is a map that charts the trans-
alpha iron can, the change from gamma iron to alpha
formation of austenite as a function of temperature and
iron causes a sudden dispersion of carbon. Because the
time and shows approximately how a particular steel
transformation from austenite is so rapid, carbon is
will respond to any rate of slow or rapid cooling from
trapped throughout the structure in this solid solution.
the austenite state. The products of this transformation
The rate of cooling is controlled by selecting an
will be discussed below.
appropriate quenching medium and cooling procedure.
PEARLITE: Austenite containing 0.83 percent of
Fresh water, brine, oil, and caustic soda in water are
carbon, cooled quickly to and held at 1,300F, does not
commonly used for rapid quenching. Slower cooling is
begin to decompose (transform) until after about 15
obtained by air cooling, by packing, and by furnace
minutes and does not completely decompose until after
cooling. Packing consists of burying the heated metal
in sand, ashes, or some other substance that is a poor
conductor of heat. Furnace cooling consists of shutting
below the critical temperature (Ac1), austenite is stable
off the heat and leaving the piece in the furnace so the
for a considerable length of time. The product of the
metal and the furnace cool together. Ferrous metals are
decomposition of austenite at this temperature is coarse
sometimes cooled in baths of molten lead or molten
pearlite of relatively low hardness. If the austenite is
15-17