1. Select a soldering bit of the proper size and
These fumes are dangerous to you and corrosive
shape for the work to be done. File and tin the
to metals.
bit if necessary.
Do not prepare zinc chloride in a closed space.
2. Heat the bit.
Hydrogen gas is released as the zinc reacts
chemically with the muriatic acid. HYDROGEN
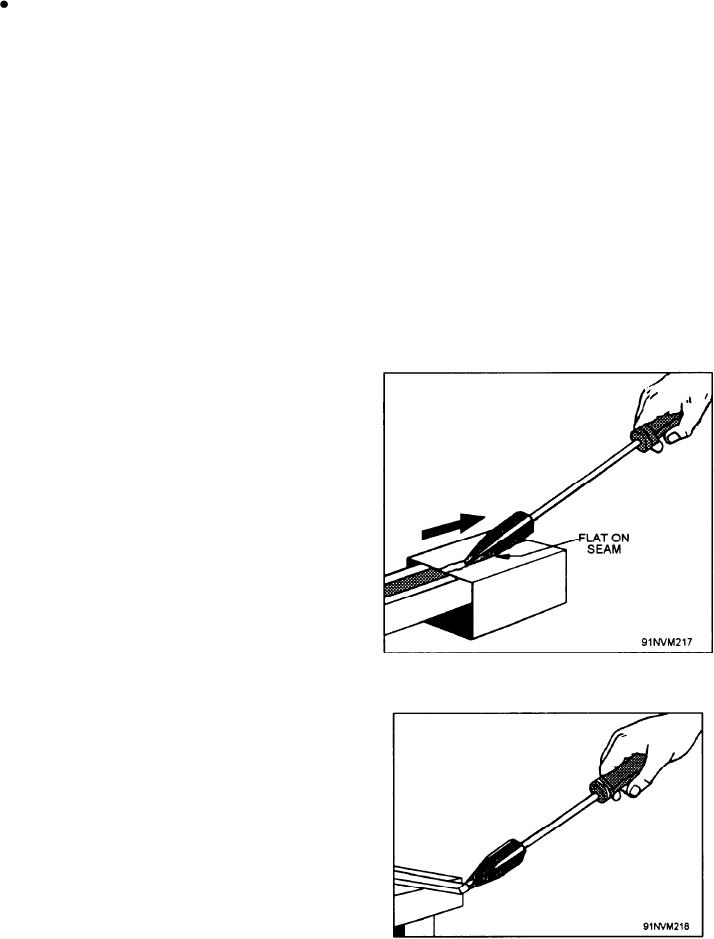
3. Position the work on a suitable support. When
IS VIOLENTLY EXPLOSIVE! Because of this,
a seam is to be soldered, position the work as
zinc chloride should always be prepared out in
shown in figure 9-27 so that the seam does not
the open or very near openings to the outside.
rest on the support. This will ensure that you
Also, take all necessary steps to prevent flames
do not lose heat to the support.
or sparks from coming in contact with the
4. Apply the flux with one or two strokes of a
released hydrogen.
brush or a swab.
Another type of corrosive flux that you may use is
5. Clean the hot soldering bit with sal ammoniac,
known as soldering salts. Commercially prepared
as described earlier in this chapter.
soldering salts are usually furnished in powder form.
The powder is dissolved in water to make a solution.
6. Touch the solder with the hot bit so that a small
When a corrosive flux has been used for soldering,
amount of solder flows over the tip of the bit,
the flux residue should be removed from the work as
as shown in figure 9-28.
completely as possible. Most corrosive fluxes are
7. Tack the pieces together, if necessary, so that
soluble in water. Wash the work with soap and water
the work will stay in position while you are
and then rinse thoroughly with clear water to remove
the residue of corrosive fluxes. Do this cleaning
immediately after you finish soldering.
Mildly corrosive fluxes, such as citric acid in water,
are sometimes used for soldering. These fluxes have
some of the advantages of the more strongly corrosive
fluxes, and some of the advantages of the noncorrosive
fluxes. The mildly corrosive fluxes clean the surfaces
of the work but do not leave a strongly corrosive
residue. These fluxes are generally used for soldering
parts that can be rinsed with water after they have been
soldered or for work in which a mildly corrosive residue
can be tolerated.
Noncorrosive fluxes are used for soldering
electrical connections and for other work that must be
completely protected from any trace of corrosive
residue. Rosin is the most commonly used noncorrosive
flux. In the solid state, rosin is inactive and
Figure 9-27.--Soldering a seam.
noncorrosive. When it is heated, it becomes active
enough to reduce the oxides on the hot metal and thus
perform the fluxing action. Rosin is available in powder,
paste, and liquid forms.
Rosin fluxes frequently leave a brown stain on the
soldered metal that is difficult to remove. You can
prevent it to some extent by adding a small amount of
turpentine to the rosin. You can also add glycerine to the
rosin to make the flux more effective.
SOLDERING WITH IRONS
When you are soldering with irons, follow this
procedure:
Figure 9-28.--Picking up solder.
9-27

