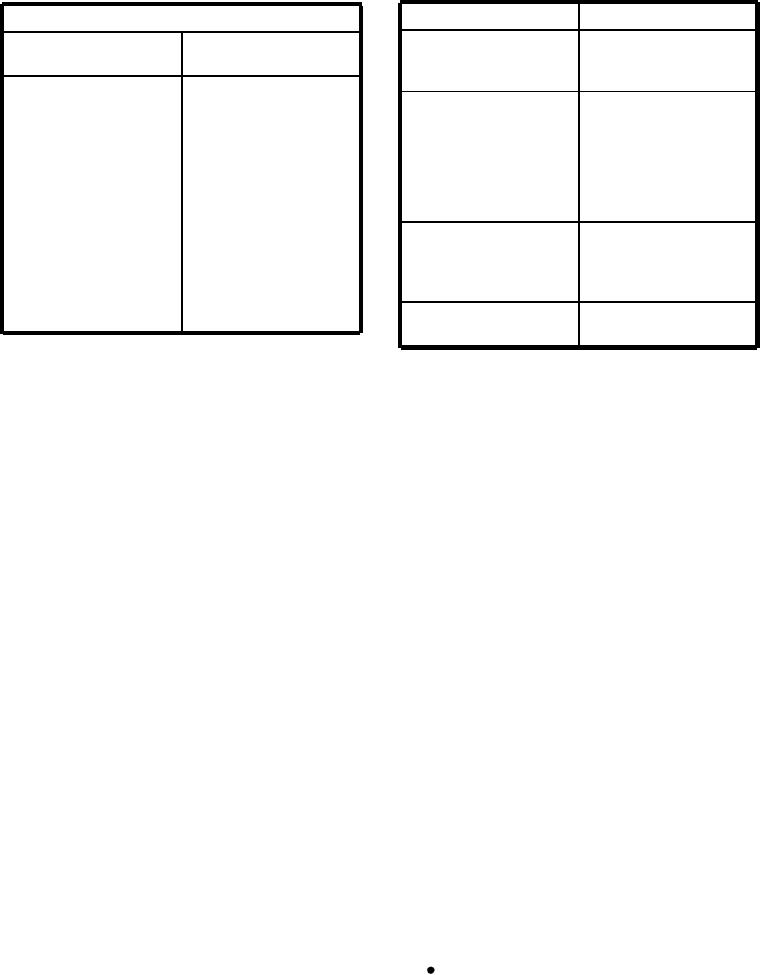
Table 9-9.--Fluxes Used for Soldering Some Common Metals
Table 9-8.--Tin-lead Melting Points
METAL SOLDERED
TIN-LEAD MELTING POINTS
Melting Point
Muriatic Acid
Galvanized Iron
(percent)
Dull Brass
(raw acid)
( F)
Dull Copper
10/90
573
Zinc Chloride
Black Iron
20/80
533
(cut acid)
Copper
Brass
30/70
496
Iron
460
40/60
Zinc
Monel
50/50
418
Tarnished Tin Plate
60/40
374
Rosin
Tin Plate
70/30
376
Lead
Bright Copper
80/20
396
Terne Plate
90/10
421
Phosphoric Acid
Stainless Steel
melting points of the metals being joined. Table 9-8
shows the melting points of most tin-lead solders.
which thorough cleaning is not possible, or where
Tin-lead solders are usually identified by numbers,
corrosion would cause a serious problem.
which indicate the percentage of tin and the percentage
The most commonly used corrosive fluxes are sal
of lead. The first number gives the percentage of tin, the
ammoniac (ammonium chloride) and zinc chloride. The
second gives the percentage of lead. For example, a
fluxes are frequently used in solution or in paste form.
30/70 solder is an alloy of 30 percent tin and 70 percent
The solvent evaporates as the work is heated, leaving a
lead. Solders containing a high percentage of tin are
layer of solid flux on the work. At the soldering
more expensive than those containing a high percentage
temperature, this layer of flux melts and partially
of lead. In general, the solders that contain a high
decomposes, releasing hydrochloric acid. The
percentage of tin have lower melting points than those
hydrochloric acid dissolves the oxides from the surface
that contain a high percentage of lead. Solders are
of the work and from the solder.
available in bars, wires, ingots, and powders. Wire
Zinc chloride (sometimes called cut acid or killed
solder is available with or without a flux core.
acid) should be made up in small amounts, as required
for use. To prepare zinc chloride, pour a small amount
FLUXES
of muriatic acid (the commercial form of hydrochloric
acid) into a container. Then, add pieces of zinc to the
To make a satisfactory joint, you must be sure that
muriatic acid until the liquid no longer boils and bubbles
the metal to be joined and the solder are free of dirt,
when the zinc is added. The zinc and the acid enter into
grease, oxides, and other foreign matter that would keep
a chemical reaction that produces zinc chloride and
the solder from adhering to the metal. Fluxes are used
hydrogen gas. When the liquid no longer boils and
to clean the joint area, to remove the oxide film that is
bubbles, the reaction is complete and the liquid in the
normally present on any metal, and to prevent further
container is no longer muriatic acid. Instead, it is a
oxidation. Fluxes also decrease the surface tension of
solution of zinc chloride in water.
the solder and thus make the solder a better wetting
agent. Table 9-9 shows the fluxes that are generally used
Strain the zinc chloride solution before using it as
with some common metals.
a flux. Any solution that is not used immediately should
Fluxes are generally classified as corrosive, mildly
be stored in a tightly sealed glass container.
corrosive, and noncorrosive. Corrosive fluxes have the
Observe the following precautions when you
best cleaning action. However, any trace of corrosive
prepare zinc chloride:
flux that remains on the work will cause corrosion of
the metal. Therefore, corrosive fluxes are not used for
Do not inhale the fumes given off by muriatic
soldering electrical connections, for other work in
acid or by the mixture of muriatic acid and zinc.
9-26

